Surgical Saw Blades
Saw blades for all bone cutting operations, compatible with all brands of surgical power tools.

Let's explain benefits of ACF Surgical Saw Blades
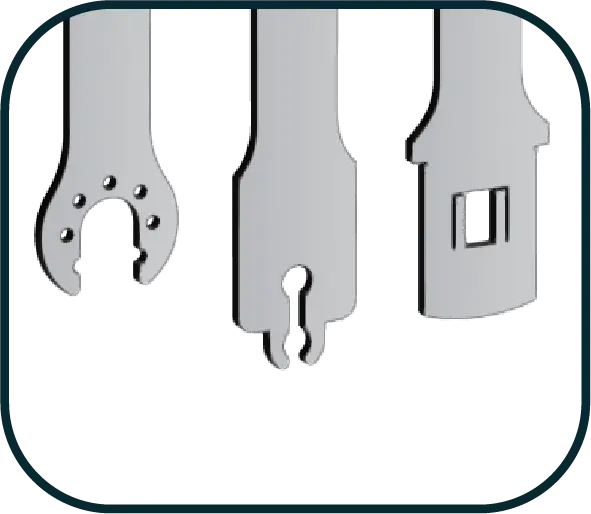
COMPATIBLE WITH ALL POWER TOOLS
Saw blades can be produced in desired sizes compatible with all brands of surgical power tools.

NON STERILE / STERILE (GAMMA) OPTIONS
The sterile process is made by the gamma irridation method with 100% sterility guarantee and has a shelf life of 5 years. It can also be produced non-sterile to reduce production time and cost.
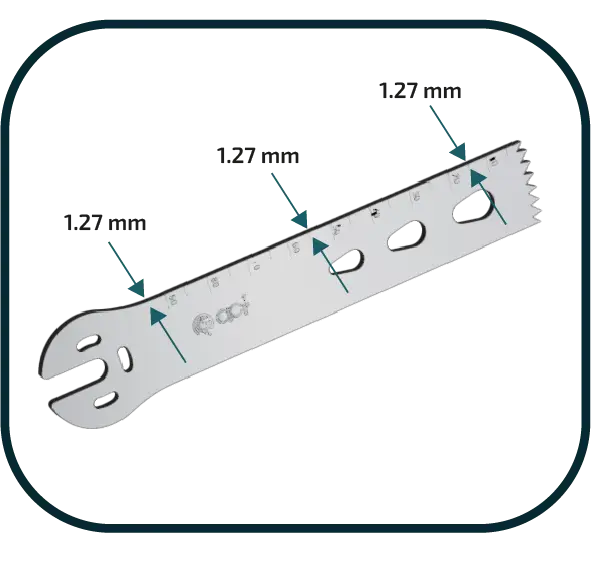
EQUAL THICKNESS ON ALL SURFACE
ACF guarantees equal thickness at every point on the blade surface.
EFFECTIVE SHARPNESS
ACF saw blades are protected their sharpness from the first to the last cut in an operation.
MOST TRUSTFUL RAW MATERIAL
Product quality begins with the raw material. Therefore choosing of the trusted/certified materials is essential.

CORRECT HARDNESS SELECTION
Correct hardness selection of raw material prevents the blade from bending or breaking, when a saw blade contacts the hard bone surface in the cutting process.
BIOCOMPATIBLE MATERIAL
Cytotoxicity Test, Intradermal Reactivity Test and Sensitization Test are repeated at each raw material changing.
ROUNDED EDGES
Rounded edges prevent any injury to users’ hands while the blade is attached or removed from surgical power tool.
